Vestibulum curae torquent diam diam commodo parturient penatibus nunc dui adipiscing convallis bulum parturient suspendisse parturient a.Parturient in parturient scelerisque nibh lectus quam a natoque adipiscing a vestibulum hendrerit et pharetra fames nunc natoque dui.
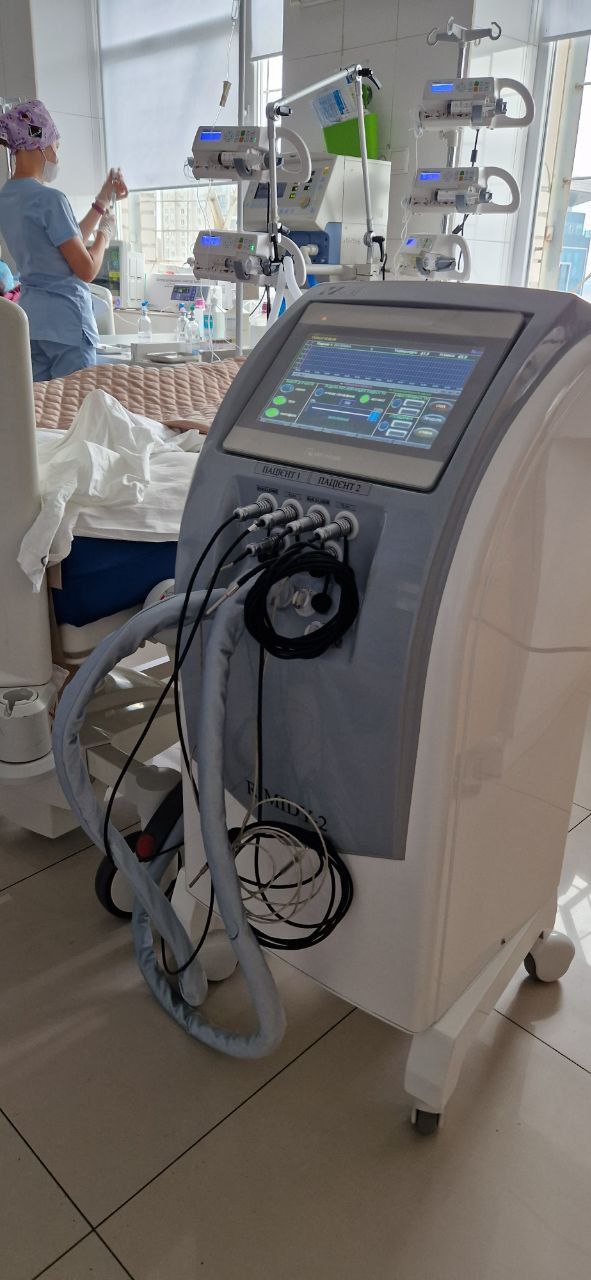
Rimidy-2 controlled hypothermia device
Rimidy-2 controlled hypothermia device designed to cool the patient’s body through the outer integuments.
One option is a heat exchange blanket, which allows you to achieve controlled General hypothermia of the entire body.
The second option is to cool the scalp and recreate local hypothermia of the skin, subcutaneous tissues, the cerebral part of the skull and structures of the human brain. Cooling of tissues is carried out by contact removal of heat from the scalp using special helmets in order to reproduce craniocerebral hypothermia (CCG).
The Rimidy-2 device is a modification of the Rimidy-1 device.
This model provides the procedure for two patients simultaneously.
The device can maintain the patient’s set body temperature and heat up to the set temperature.
The heat carrier is distilled water.
Scope of application of the device:
– psychiatry;
– Narcology;
– neurosurgery;
– obstetrics and gynecology;
– oncology;
– Traumatology.
Technical specifications:
Overall dimensions: 420mm / 400mm / 1000mm (L / W / H)
device weight 55 kg
In terms of Electrical Safety, the device is made according to protection class I with a working part according to the degree of protection against electric shock according to type BF DSTU en 60601-1.
The device is operational when powered from an AC Network with a frequency of 50 Hz, voltage (220 ± 10 %) V. The rated power consumed by the device is 1000 VA.
The time to prepare the device for operation (from the moment the “network” switch is pressed to the start of operation) is no more than 5 minutes.
The device provides an indication:
– digital-coolant temperature, patient temperature;
– symbolic-levels of heat carrier in the tanks,
– sound-start and end of an emergency by pressing a button on the control panel;
– light-indicates the presence of power supply from the mains when the device is turned on.
The temperature setting range is from plus 5 ° C to plus 50 ° C.
Composition and operation
The principle of operation of the device is as follows. cooled or heated water (depending on the given type of exposure) is fed through hoses to the helmet or blanket that comes into contact with the patient and thus affects the biological tissue
The control and display unit performs the general functioning of the device and provides:
– sensor test;
– temperature measurements of the patient/each of the two patients;
– cooling of the heat carrier;
– heating of the heat carrier;
– fluid supply to the patient’s helmet (blanket or pad) ;
– automatic monitoring of patient and heat carrier temperatures;
– execution of time parameters of the cycle.
On the front panel of the control unit there is a touch-screen information display that displays the progress of the hypothermia process, the occurrence of emergencies and has buttons for starting/stopping the process and turning on/off the device.
The information display shows:
- * name of the selected mode (heating or cooling);
- * current stage of the process;
- * process duration (time until the end of the current process);
- * tank temperature;
- * current temperature of the patient/each of the two patients;
- * liquid levels in tanks;
- * AKG status.
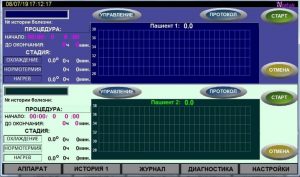
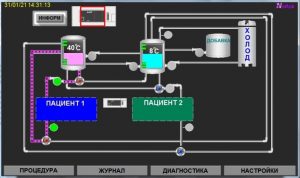
Hardware status
Maintaining the temperature of patients ‘ bodies is carried out by the signals of the leading ones – the main sensors (ear), and in case of their failure, auxiliary sensors (rectal). The formation of control commands to ensure changes in patient body temperature measured by wired sensors is carried out in accordance with the initial data specified in the protocols with an accuracy of 0.2 °C
On removable media (USB Flash) stored:
– procedure protocols for 50 patients;
– data of the procedure process (temperature and time for each patient), which is stored for 7 days;
– all events that occur during the process are recorded in the event log and this information is stored for 7 days.


MAECENAS IACULIS
ADIPISCING CONVALLIS BULUM
- Vestibulum penatibus nunc dui adipiscing convallis bulum parturient suspendisse.
- Abitur parturient praesent lectus quam a natoque adipiscing a vestibulum hendre.
- Diam parturient dictumst parturient scelerisque nibh lectus.
Scelerisque adipiscing bibendum sem vestibulum et in a a a purus lectus faucibus lobortis tincidunt purus lectus nisl class eros.Condimentum a et ullamcorper dictumst mus et tristique elementum nam inceptos hac parturient scelerisque vestibulum amet elit ut volutpat.
Related products
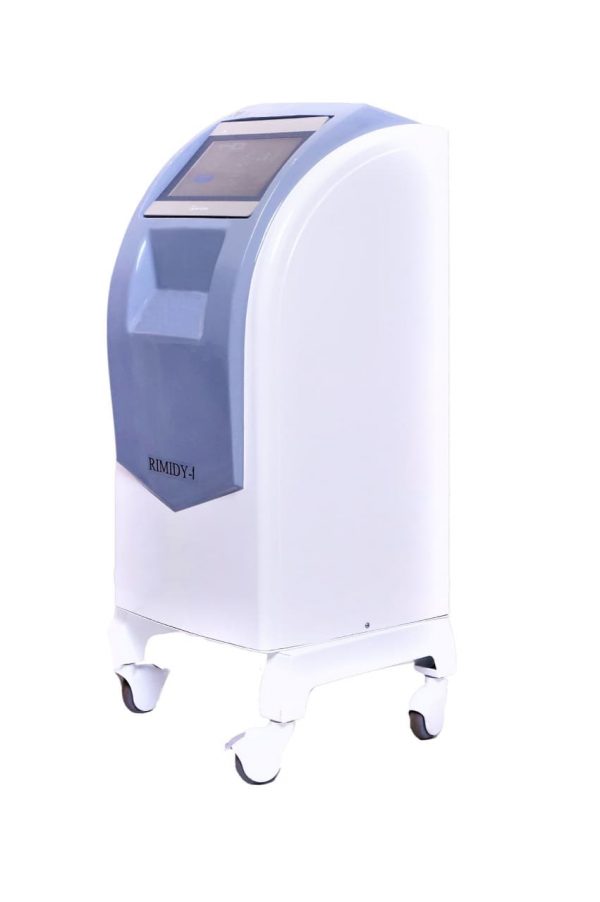
Rimidy-1 controlled hypothermia device
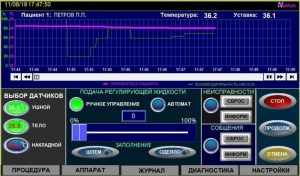 Visual display of the procedure completion
Maintaining the temperature of patients ‘ bodies is carried out by the signals of the leading ones – the main sensors (ear), and in case of their failure, auxiliary sensors (rectal). The formation of control commands to ensure changes in patient body temperature measured by wired sensors is carried out in accordance with the initial data specified in the protocols with an accuracy of 0.2 °C.
On removable media (USB Flash) stored:
– procedure protocols for 50 patients;
– data of the procedure process (temperature and time for each patient), which is stored for 7 days;
– all events that occur during the process are recorded in the event log and this information is stored for 7 days.
Visual display of the procedure completion
Maintaining the temperature of patients ‘ bodies is carried out by the signals of the leading ones – the main sensors (ear), and in case of their failure, auxiliary sensors (rectal). The formation of control commands to ensure changes in patient body temperature measured by wired sensors is carried out in accordance with the initial data specified in the protocols with an accuracy of 0.2 °C.
On removable media (USB Flash) stored:
– procedure protocols for 50 patients;
– data of the procedure process (temperature and time for each patient), which is stored for 7 days;
– all events that occur during the process are recorded in the event log and this information is stored for 7 days.  +38(095)290 08 98
+38(095)290 08 98  vostv.medgrup23@gmail.com
vostv.medgrup23@gmail.com






Reviews
There are no reviews yet.